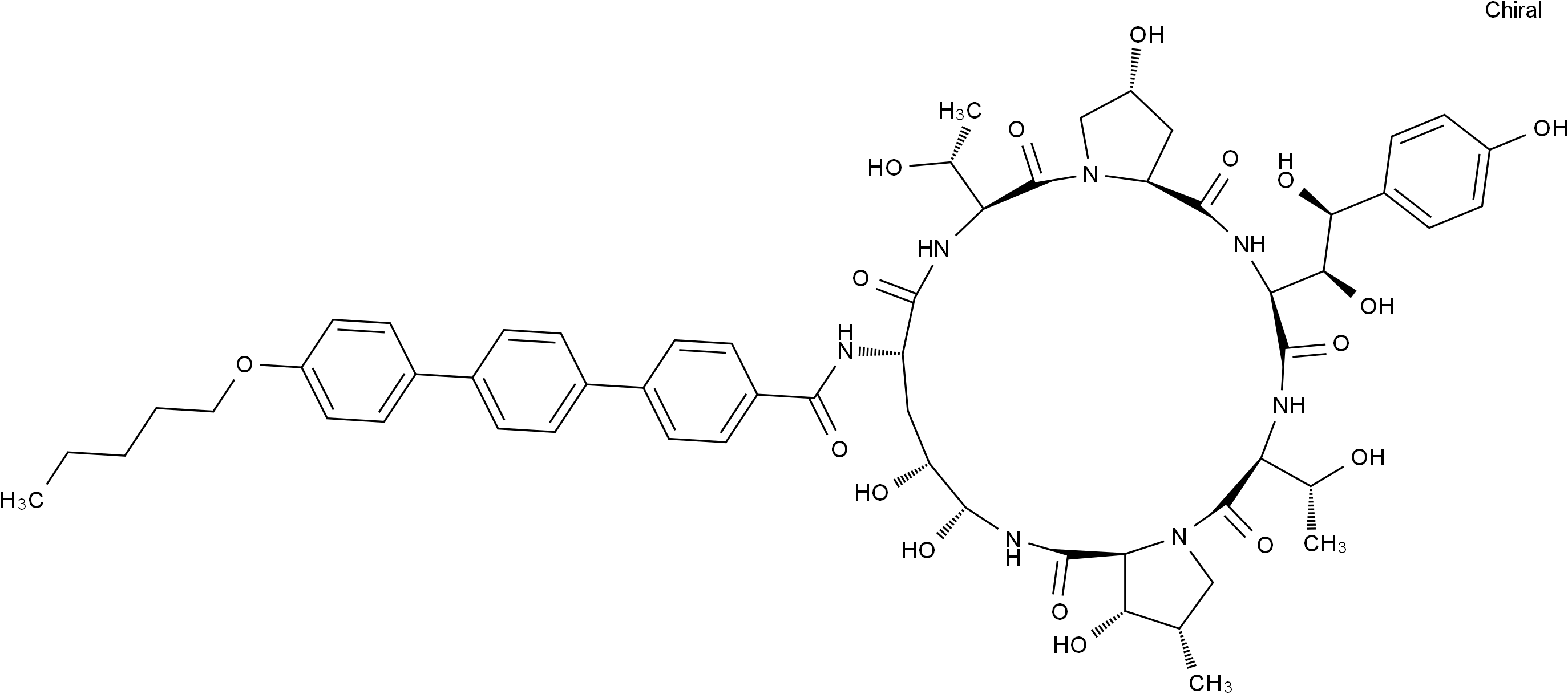
Anidulafungin
CAS No. 166663-25-8
Anidulafungin ( LY303366 )
Catalog No. M12541 CAS No. 166663-25-8
Anidulafungin (LY303366), an echinocandin derivative, inhibits glucan synthase activity, used as an antifungal drug.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 30 | In Stock |


|
| 5MG | 47 | In Stock |


|
| 10MG | 88 | In Stock |


|
| 25MG | 152 | In Stock |


|
| 50MG | 235 | In Stock |


|
| 100MG | 329 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameAnidulafungin
-
NoteResearch use only, not for human use.
-
Brief DescriptionAnidulafungin (LY303366), an echinocandin derivative, inhibits glucan synthase activity, used as an antifungal drug.
-
DescriptionAnidulafungin (LY303366), an echinocandin derivative, inhibits glucan synthase activity, used as an antifungal drug.(In Vitro):Anidulafungin (LY-303366) has MICs of ≤0.32 μg/mL for all Candida albicans (n=99), Candida glabrata (n=18), and Candida tropicalis (n=10) isolates tested. Anidulafungin is also active against Aspergillus species (minimum effective concentration at which 90% of the isolates are inhibited, 0.02 μg/mL) (n=20), is less active against Candida parapsilosis (MIC at which 90% of the isolates are inhibited [MIC90], 5.12 μg/mL) (n=10), and is inactive against C. neoformans (MIC90 >10.24 μg/mL) (n=15) and B. dermatitidis (MIC90, 16 μg/mL) (n=29).The MICs of Fluconazole for three strains of C. tropicalis, seven strains of C. glabrata, and two strains of Candida krusei are ≥16 μg/mL. The MICs of Anidulafungin for 11 of these 12 strains range from 0.08 to 0.32 mg/mL. The twelfth strain is a C. krusei strain (Fluconazole MIC, 32 μg/mL) for which the Anidulafungin MIC is 1.28 mg/mL. The MIC at which 90% of the isolates are inhibited (MIC90) for these 12 strains is 0.32 μg/mL. The Anidulafungin MIC90 for the remaining 18 C. glabrata isolates and C. tropicalis isolates for which the Fluconazole MICs are ≥ 8 μg/mL is also 0.32 mg/mL. Anidulafungin appeares equally active against Candida species for which the fluconazole MICs are ≥16 mg/mL and against those for which the fluconazole MICs are ≥ 8 μg/mL. Anidulafungin has significantly less activity against C. neoformans and B. dermatitidis than against C. albicans, C. glabrata, and C. tropicalis. Ketoconazole and amphotericin B are the most active antifungal agents tested for both C. neoformans and B. dermatitidis. Anidulafungin demonstrated potent in vitro activity against Aspergillus species with a MEC90 of 0.02 μg/mL. MICs of Anidulafungin for the control strain yeast isolates are 0.02 μg/mL for C. albicans ATCC 90028, 0.16 mg/mL for C. glabrata ATCC 90030, and >10.24 μg/mL for C. neoformans ATCC 90112. Strains selected with CD101 that have a 2-fold or greater CD101 MIC increase also have at least a 2-fold MIC increase for Anidulafungin (ANF) and/or Caspofungin (CSF).
-
In VitroAnidulafungin (LY-303366) has MICs of ≤0.32 μg/mL for all Candida albicans (n=99), Candida glabrata (n=18), and Candida tropicalis (n=10) isolates tested. Anidulafungin is also active against Aspergillus species (minimum effective concentration at which 90% of the isolates are inhibited, 0.02 μg/mL) (n=20), is less active against Candida parapsilosis (MIC at which 90% of the isolates are inhibited [MIC90], 5.12 μg/mL) (n=10), and is inactive against C. neoformans (MIC90 >10.24 μg/mL) (n=15) and B. dermatitidis (MIC90, 16 μg/mL) (n=29).The MICs of Fluconazole for three strains of C. tropicalis, seven strains of C. glabrata, and two strains of Candida krusei are ≥16 μg/mL. The MICs of Anidulafungin for 11 of these 12 strains range from 0.08 to 0.32 mg/mL. The twelfth strain is a C. krusei strain (Fluconazole MIC, 32 μg/mL) for which the Anidulafungin MIC is 1.28 mg/mL. The MIC at which 90% of the isolates are inhibited (MIC90) for these 12 strains is 0.32 μg/mL. The Anidulafungin MIC90 for the remaining 18 C. glabrata isolates and C. tropicalis isolates for which the Fluconazole MICs are ≥ 8 μg/mL is also 0.32 mg/mL. Anidulafungin appeares equally active against Candida species for which the fluconazole MICs are ≥16 mg/mL and against those for which the fluconazole MICs are ≥ 8 μg/mL. Anidulafungin has significantly less activity against C. neoformans and B. dermatitidis than against C. albicans, C. glabrata, and C. tropicalis. Ketoconazole and amphotericin B are the most active antifungal agents tested for both C. neoformans and B. dermatitidis. Anidulafungin demonstrated potent in vitro activity against Aspergillus species with a MEC90 of 0.02 μg/mL. MICs of Anidulafungin for the control strain yeast isolates are 0.02 μg/mL for C. albicans ATCC 90028, 0.16 mg/mL for C. glabrata ATCC 90030, and >10.24 μg/mL for C. neoformans ATCC 90112. Strains selected with CD101 that have a 2-fold or greater CD101 MIC increase also have at least a 2-fold MIC increase for Anidulafungin (ANF) and/or Caspofungin (CSF).
-
In Vivo——
-
SynonymsLY303366
-
PathwayOthers
-
TargetOther Targets
-
Recptorglucan synthase
-
Research AreaInfection
-
Indication——
Chemical Information
-
CAS Number166663-25-8
-
Formula Weight1140.24
-
Molecular FormulaC58H73N7O17
-
Purity>98% (HPLC)
-
SolubilityDMSO:100 mg/mL warmed (87.7 mM); Ethanol:<1 mg/mL warmed (<1 mM); Water:<1 mg/mL (<1 mM)
-
SMILESCCCCCOC1=CC=C(C2=CC=C(C3=CC=C(C(N[C@@H]4C[C@@H](O)[C@@H](O)NC([C@@H]5[C@@H](O)[C@@H](C)CN5C([C@H]([C@@H](O)C)NC([C@H]([C@@H](O)[C@@H](O)C6=CC=C(O)C=C6)NC([C@@H]7C[C@@H](O)CN7C([C@H]([C@@H](O)C)NC4=O)=O)=O)=O)=O)=O)=O)C=C3)C=C2)C=C1
-
Chemical NameN-((2R,6S,9R,11R,12R,14aS,15S,16S,20S,23S,25aS)-23-((1R,2S)-1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl)-2,11,12,15-tetrahydroxy-6,20-bis((S)-1-hydroxyethyl)-16-methyl-5,8,14,19,22,25-hexaoxotetracosahydro-1H-dipyrrolo[2,1-c:2',1'-l][1,4,7,10,13,16]hexaazacyclohenicosin-9-yl)-4''-(pentyloxy)-[1,1':4',1''-terphenyl]-4-carboxamide.
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Yá?ez L, et al. Transpl Infect Dis. 2015 Oct;17(5):761-7.
molnova catalog



related products
-
Gynosaponin TN2
Gynosaponin TN-2 has anti-Parkinsonian activity. Gynosaponin TN-2 exerts protective effects on L-DOPA (100 and 200 μM)-induced apoptotic cell death by modulating extracellular signal-regulated protein kinases 1 and 2 activation in pheochromocytoma 12 cells.
-
Metaldehyde
Metaldehyde (Metacetaldehyde) is an agriculturally used molluscicide that induces DNA fragmentation in cells.
-
Ponsegromab
Ponsegromab (PF 06946860) is a selective and effective humanized anti-GDF15 antibody inhibitor with anti-cachexic activity.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


